Chapter 20 Generalised linear models
The models we have covered so far are ordinary linear models (including ANOVA, ANCOVA, ordinary linear regression etc.) that assume that the relationship between the explanatory variables and the response variable is linear, and that the systematic error in the model is constant (homoscedastic, i.e. the standard deviation of the data does not depend on the magnitude of the explanatory variable).
In many cases this will not be the case. Non-linearity and heteroscedasticity tend to go hand-in-hand. Sometimes, for example, the data show an exponential growth type of pattern, and/or may be bounded in such a way that the errors cannot be homoscedastic. For example, counts of number of species on islands of different sizes have a lower bound at zero (you can’t have negative numbers of species!) and increase exponentially while the standard deviations are small for small islands and large for large islands. Ratio data or percentage data such as proportion of individuals dying/surviving is bounded between 0 and 1 (0 - 100%).
Transformation of the response variable could be an option to linearise these data (although there would be problems with 0 values because log(0) = -Infinity), but a second problem is that the ordinary linear model assumes “homoscedasticity” - that the errors in the model are evenly distributed along the explanatory variables. This assumption will be violated in most cases. For example, with count data (e.g. number of species in relation to island size), the errors for very small islands will be smaller than those for large islands. In fact, even if we transform the response variable, for example by log transformation, the predictions of the model will allow errors that include negative counts. This is clearly a problem!
Generalised linear models (GLMs) solve these problems by not only applying a transformation but also explicitly altering the error assumption. They do this using a link function to carry out the transformation and by choosing an error structure (sometimes referred to as family, or variance function). The choice of link and error structure can be a bit confusing, but there are so-called “canonical links” that are commonly associated with particular error structures. For example, a model for count data would usually have a log link and a Poisson error structure.
The flexibility of the GLM approach means that one can fit GLM versions of all of the models you have already learned about until this point: ANOVA-like GLMs, ANCOVA-like GLMs, ordinary regression-like GLMs and so on.
In this chapter I will focus on count data, and in the next chapter I will broaden the focus to illustrate uses of other data types.
20.1 Count data with Poisson errors.
The most common kind of count data where Poisson errors would be expected are frequencies of an event: we know how many times an event happened, but not how many times it did not happen (e.g. births, deaths, lightning strikes).
In these cases:
- Linear model could lead to negative counts.
- Variance of response likely to increase with mean (it usually does with count data).
- Errors are non-normal.
- Zeros difficult to deal with by transformation (e.g. log(0) = -Inf).
- Other error families do not allow zero values.
The standard (“canonical”) link used with the Poisson error family is the log link. The log link ensures that all fitted (i.e. predicted) values are positive, while the Poisson errors take account of the fact that the data are integer and the variance scales 1:1 with the mean (i.e. variance increases linearly and is equal to the mean).
There are other potential link and error families that could be used with this kind of data, but we’ll stick with the standard ones here. Let’s look at a couple of examples…
20.1.1 Example: Number of offspring in foxes.
This example uses the fox.csv data set. This data set gives the number of offspring produced by a group of foxes, alongside the weight (in kg) of the mothers. Let’s import and plot the data.
Remember to load the dplyr, magrittr and
ggplot packages, and to set your working directory
correctly.
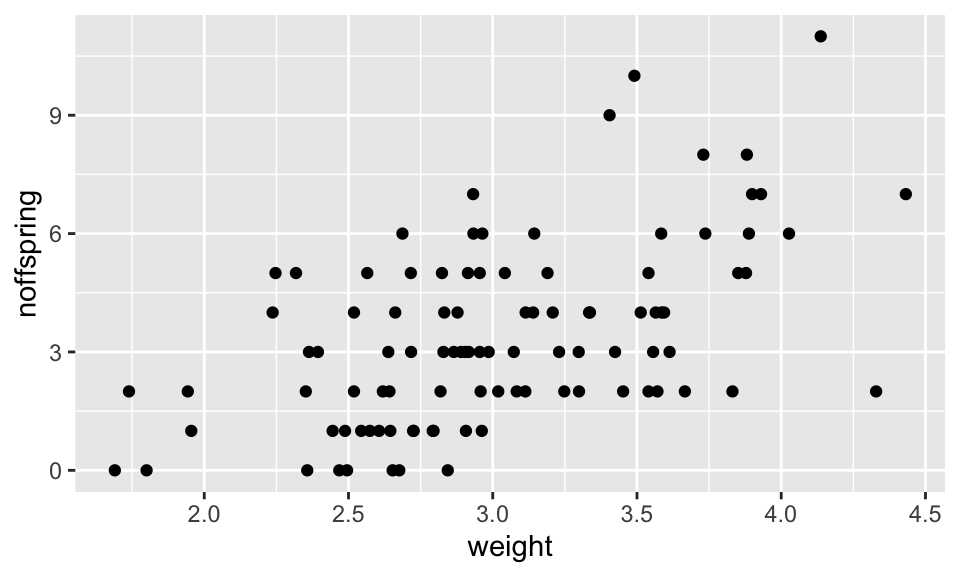
The first thing to notice is that, like all count data, the data form horizontal rows reflecting the fact that the response values are integers. There is clearly an increasing pattern, but how can we formally test for a statistical relationship? It is obvious that fitting an ordinary linear model to these data would not be the right approach: this would lead to the prediction of negative numbers of offspring for small foxes, and the variance appears to increase with weight/number of offspring. Therefore this is a good candidate for a GLM. The data are bounded at 0 and are integer values, so the usual approach is to fit a GLM with Poisson errors (and the standard log link).
After fitting the model it is a good idea to look at the model diagnostics, using autoplot from the ggfortify package.
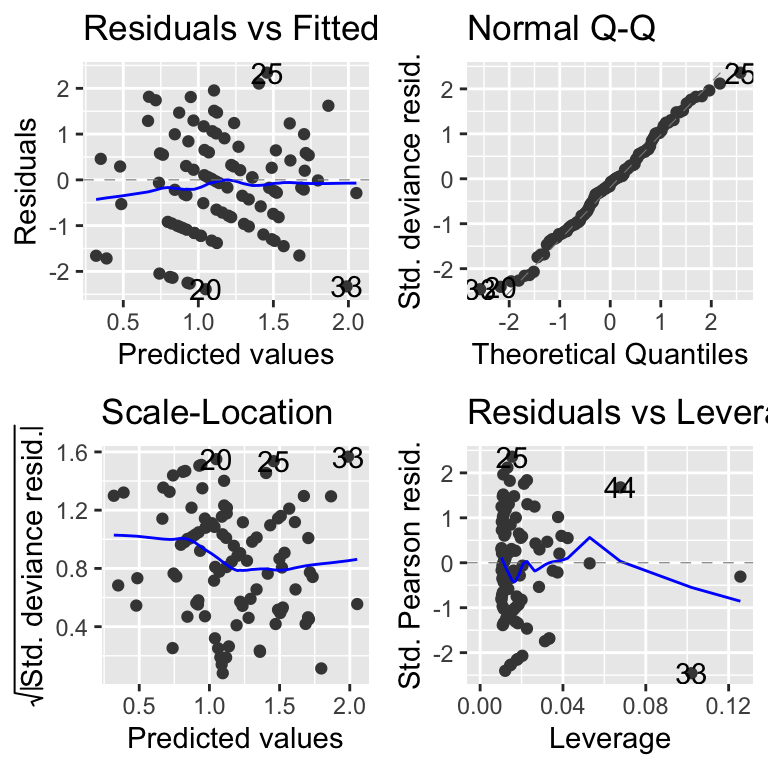
These diagnostic plots are basically the same as the ones you saw for lm models. There is one difference: the plots use standardised deviance residuals instead of standardised residuals. These are transformed versions of the residuals that will look normal (in the statistical sense) if the family of the GLM is appropriate. So, if “poisson” is the right family, the QQ-plot should show points on the diagonal dashed line, and the Scale-location plot should have no strong patterns.
Now we can ask for the Analysis of Variance table for this model. This is exactly the same procedure as for the previous linear models (ANOVA, ANCOVA etc.) except for GLMs you must also specify that you would like to see the results of significance tests using test = "F" or test = "Chi". For Poisson and binomial GLMs the chi-squared test is most appropriate, while for Gaussian (normal), quasibinomial and quasipoisson models the F test is most appropriate.
## Analysis of Deviance Table
##
## Model: poisson, link: log
##
## Response: noffspring
##
## Terms added sequentially (first to last)
##
##
## Df Deviance Resid. Df Resid. Dev Pr(>Chi)
## NULL 99 166.84
## weight 1 44.124 98 122.72 3.082e-11 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1This summary table tells us that the single explanatory variable (weight) is fantastically important (p-value is very small indeed).
We can then ask for the coefficient summary using summary.
##
## Call:
## glm(formula = noffspring ~ weight, family = poisson, data = fox)
##
## Coefficients:
## Estimate Std. Error z value Pr(>|z|)
## (Intercept) -0.74981 0.31107 -2.410 0.0159 *
## weight 0.63239 0.09502 6.655 2.83e-11 ***
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## (Dispersion parameter for poisson family taken to be 1)
##
## Null deviance: 166.85 on 99 degrees of freedom
## Residual deviance: 122.72 on 98 degrees of freedom
## AIC: 405.56
##
## Number of Fisher Scoring iterations: 5GLM model coefficients and predicted values, are expressed on the scale of the linear predictor (i.e. the transformed data scale). It is usually desirable to “backtransform” to the natural scale before plotting. See below.
The model coefficients and their standard errors are given on the scale of the linear predictor. They tell us that there is a significant association between the weight of the fox mother and the number of offspring she will produce: larger foxes produce more offspring. Because the coefficients are given on the scale of the linear predictor rather than on the real scale it is useful to plot predictions of the model to visualise the relationship.
To do that we can use the following four steps:
tell the model what to predict
fromi.e. we must provide a suitable set of values to predict from, In this case, we do that using theseqfunction to create a vector in a data frame.use the
predictfunction to predict values (fit) from the model. We add the argumentse.fit = TRUEto tell thepredictfunction to give us the standard error estimates of the fit.we then put these values into the
newDatadata frame. These.fitvalues are added or subtracted from the fit to obtain the plus/minus standard errors. We can multiply these by 1.96 to get the 95% confidence intervals of the fitted values.at this point, the predicted values (and the errors) are be on the scale of the linear predictor (which is why I have given them the “_LP” suffix). We must now back-transform them to the natural scale using the appropriate “inverse” of the link function. We can extract this function from the model object with
family(MODEL_NAME)$linkinv. We can then apply that function to the predicted data.
That’s a lot! Let’s put it all together. It is worth saving the code somewhere so you can cut-and-paste it (with some modification) for future use.
# (1) Create a data frame with a vector to predict from
newData <- data.frame(weight = seq(1.7, 4.4, 0.01))
# (2) Use predict, to get the predicted values (and SE)
predVals <- predict(mod1, newData, se.fit = TRUE)
# (3) Add the predicted fit and SE to newData
newData <- newData %>%
mutate(fit_LP = predVals$fit) %>%
mutate(lowerCI_LP = predVals$fit - 1.96 * predVals$se.fit) %>%
mutate(upperCI_LP = predVals$fit + 1.96 * predVals$se.fit)
# (4) Backtransform these data to the natural scale
# Get the inverse link function
inverseFunction <- family(mod1)$linkinv
# transform predicted data to the natural scale
newData <- newData %>%
mutate(
fit = inverseFunction(fit_LP),
lowerCI = inverseFunction(lowerCI_LP),
upperCI = inverseFunction(upperCI_LP)
)Take a look at this data to make sure it looks OK.
## weight fit_LP lowerCI_LP upperCI_LP fit lowerCI upperCI
## 1 1.70 0.3252609 0.02226977 0.6282521 1.384392 1.022520 1.874332
## 2 1.71 0.3315849 0.03033712 0.6328326 1.393174 1.030802 1.882937
## 3 1.72 0.3379088 0.03840303 0.6374146 1.402013 1.039150 1.891584
## 4 1.73 0.3442328 0.04646747 0.6419981 1.410907 1.047564 1.900274
## 5 1.74 0.3505567 0.05453044 0.6465830 1.419858 1.056045 1.909007
## 6 1.75 0.3568807 0.06259189 0.6511695 1.428865 1.064592 1.917782This looks OK. Now we can plot the data and add a the model fit line, and a “ribbon” representing the errors (the 95% confidence interval for the line). Because the data used to draw the ribbon are not from the original dataset, we need to tell the geom_ribbon function NOT to use the same aesthetics (inherit.aes = FALSE). The geom layers are applied sequentially, so I add the real points last so they are not obscured by the ribbon or line.
(A <- ggplot(fox, aes(x = weight, y = noffspring)) +
geom_ribbon(data = newData,
aes(x = weight, ymin = lowerCI, ymax = upperCI),
fill = "grey75",
inherit.aes = FALSE) +
geom_line(data = newData, aes(x = weight, y = fit)) +
geom_point())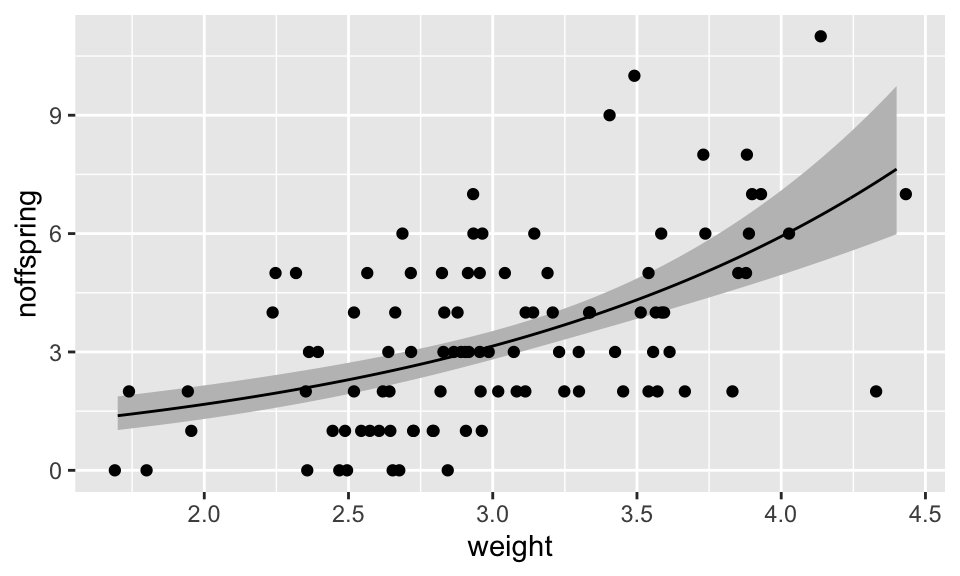
As you can see, this procedure is long-winded and error-prone. I therefore recommend that you save this code somewhere so you can cut-and-paste it (with some modifications) for future use!
So we could summarise this something like this:
Methods: I modelled the association between mother’s weight and number of pups produced using a generalised linear model with a log link and Poisson error structure. This is appropriate because the data are count data (number of pups) that are bounded at 0 with increasing variance with increased maternal weight.
Results: The GLM showed that maternal weight was significantly associated with the number of pups produced (GLM: Null Deviance = 166.8, Residual Deviance = 122.7, d.f. = 1 and 98, p <0.001). The slope of the relationship was 0.63 (on the log scale). The equation of the best fit line was log(nOffspring) = -0.75 + 0.63 \(\times\) MotherWeight (see Figure XXX)
20.1.2 Example: Cancer clusters
This data show counts of prostate cancer and distance from a nuclear processing plant. Lets take a look at the data.
Let’s first import the data (cancer.csv) and use summary to examine it by plotting it:
First we can see that there are no negative count values.
cancer <- read.csv("CourseData/cancer.csv")
ggplot(cancer, aes(x = Distance, y = Cancers)) +
geom_point()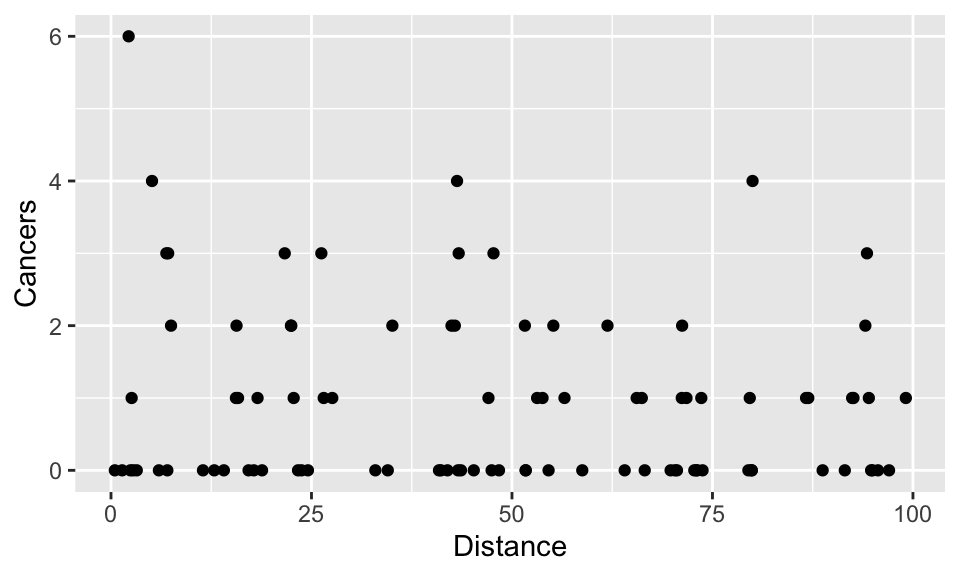
Again, you will notice that the data are formed into horizontal rows of integer response values. There are lots of zero values at all distances, but the biggest cluster (6 cases), is very close to the plant. But is there a relationship between the distance from the nuclear plant and the number of cancers?
Let’s fit a Generalised Linear Model to find out. As before will assume that the error is Poisson (that they variance increases directly in proportion to the mean), and we will use the standard log link to ensure that we don’t predict negative values:
Next, plot the diagnostic plots.
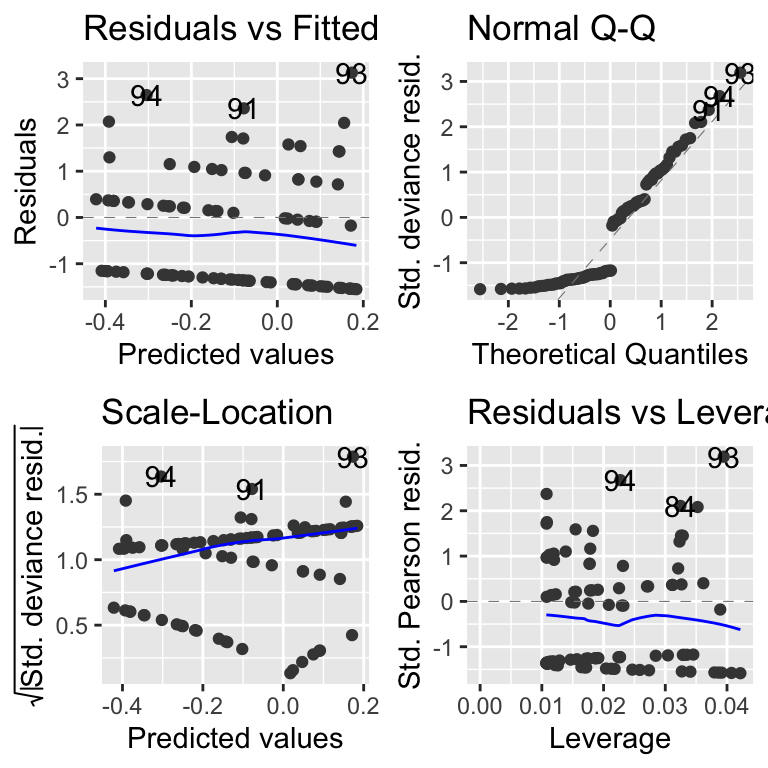
These look a bit dodgy, but we’ll stick with it for the moment.
Next ask for the Analysis of Variance table.
## Analysis of Deviance Table
##
## Model: poisson, link: log
##
## Response: Cancers
##
## Terms added sequentially (first to last)
##
##
## Df Deviance Resid. Df Resid. Dev Pr(>Chi)
## NULL 93 149.48
## Distance 1 2.8408 92 146.64 0.0919 .
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1The ANOVA table tells us that there is no significant effect of the Distance variable. In other words a model that includes the Distance term does not explain significantly more variation than the NULL model that includes no terms and instead assumes that variation in cancer incidence is simply caused by random variation.
We needn’t go further with this model, but go ahead and plot the model in any case (just for practice).
##
## Call:
## glm(formula = Cancers ~ Distance, family = poisson, data = cancer)
##
## Coefficients:
## Estimate Std. Error z value Pr(>|z|)
## (Intercept) 0.186865 0.188728 0.990 0.3221
## Distance -0.006138 0.003667 -1.674 0.0941 .
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## (Dispersion parameter for poisson family taken to be 1)
##
## Null deviance: 149.48 on 93 degrees of freedom
## Residual deviance: 146.64 on 92 degrees of freedom
## AIC: 262.41
##
## Number of Fisher Scoring iterations: 5Use the approach from the fox example as guidance to make a plot with a fit line.
20.1.3 Overdispersion: What It Is and Why It Matters
Overdispersion occurs when the observed variance in the response variable exceeds what is expected under the model’s assumptions. For example, in a Poisson model, the variance is expected to equal the mean. If the actual variance is significantly larger, overdispersion is present. There’s a nice intuitive description of this in the textbook (Getting Started With R, section 7.5.1). To diagnose overdispersion, you compare the Residual deviance to the degrees of freedom. In a perfect Poisson model, these values will be equal, so we can generate a useful index of overdispersion by dividing the residual deviance by the degrees of freedom. The “rule of thumb” is that an overdispersion index >2 is problematic.
Causes of Overdispersion:
- Unobserved Heterogeneity: Variability in the data that is not captured by the predictors in the model (e.g., different groups or subpopulations behaving differently).
- Clustering or Correlation: When observations are not independent but are instead grouped or correlated (e.g., measurements from the same individual or location).
- Excess Zeros or Outliers: An unusually high number of zero observations or extreme values in the data.
Why Overdispersion Matters: If overdispersion is ignored, standard errors of the estimated coefficients will be underestimated, leading to overly optimistic confidence intervals and \(p\)-values. This increases the risk of drawing incorrect conclusions about the effects of predictors.
What to do about overdispersion
The solution to overdispersion in a GLM framework is often to use a quasi-likelihood model. For count data, this corresponds to using the quasipoisson family (family = quasipoisson). With this family, the model works in the same way as the regular poisson model, except that it does not assume the variance equals the mean (as in the Poisson case). Instead, it allows the variance to increase as a function of the mean, estimating a dispersion parameter that accounts for overdispersion. This adjustment affects the standard errors and p-values but does not alter the coefficient estimates.
Another approach to addressing overdispersion is to use a negative binomial model, which can be fitted using the glm.nb function from the MASS package. This model introduces an additional parameter that explicitly models the relationship between the mean and variance, allowing the variance to grow quadratically with the mean.
A further, slightly more involved option, is to use a zero-inflated model, which is particularly useful when overdispersion arises due to an excessive number of zeros in the data. These models assume the data-generating process has two components: one generating the zeros (often modelled with a logistic component) and another generating the counts (often modelled as Poisson or negative binomial). Zero-inflated models can be fitted using specialized functions such as zeroinfl from the pscl package.
While these methods require slightly different implementation, they are conceptually similar to regular GLMs and are interpreted in comparable ways.
20.2 Exercise: Maze runner
In an experiment, researchers studied the ability of children and adults to navigate through a maze. They recorded the number of mistakes each person made before successfully completing the maze. The data (maze.csv) has two columns: Age (a categorical variable with two levels - Adult and Child) and nErrors a count of the number of errors that each subject makes.
In this example, you will be fitting a GLM equivalent of a t-test that is appropriate for count data.
- Import the data and graph it (
geom_boxplot). Try adding the points to theggplotusing the new (to you) functiongeom_dotplot(binaxis = "y", stackdir = "center"). - Fit an appropriate GLM.
- Examine the diagnostic plots of the model (
autoplot). - Get the analysis of variance (deviance) table (
anova). What does this tell you? - Obtain the
summarytable. What does this tell you? - Use the coefficient information in the
summarytable to get the model predictions for average number of mistakes (plus/minus 95% Confidence interval). Remember that (i) the model summary is on the scale of the linear predictor, and (ii) the 95% CI can be calculated as 1.96 times the standard error. You can do these calculations “by hand”, or using thepredictfunction. Ask for help if you get stuck.